Anodic Protection Systems Performance
Anodic protection is a type of corrosion protection of metals by stabilizing the passive oxide layer on their surface. In this method, in contrast to the cathodic protection method that is applied current into structure, the current is stretched from structure until the passive metal layer is stabilized, then current stretching continues at a very low rate to maintain the stability of the passive layer and to reduce its corrosion to a minimum.
The tendency for anodic polarization behavior is due to the formation of a surface layer on the metal that is relatively insoluble in the chemical environment. In fact, passivation results in high corrosion resistance of the metal. So anodic protection on the one hand can be used to form passive films on metals in chemical systems that are generally corrosive, and on the other hand to maintain passive metals that may be altered to active state and corroded in a process to be used. In other words, the success of this method of protection requires that the metal in a corrosive environment be capable of forming a passive layer with chemical composition, temperature, concentration, velocity and impurities.
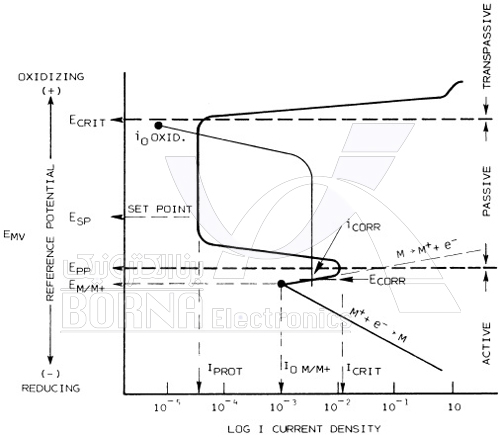
Polarization curve of an active-passive metal
Metals and solutions susceptible to anodic protection
| Metals | Solutions |
|
Steels Stainless Steels Nickel Nickel Alloys Chromium |
Sulfuric Acid Phosphoric Acid Nitric Acid Nitrate solutions Aqueous ammonia Organic acids Caustic solutions |
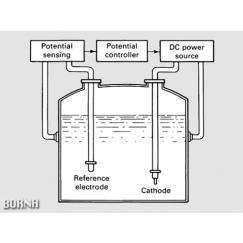
To anodic protection of a structure, a device called potentiostat is used. Potentiostat is an electronic device that can hold the metal at a constant potential relative to the reference electrode. Potentiostat has three outputs, one connected to the structure, the other to the cathode, and the third to the reference electrode. In practice, the potentiostat maintains a constant potential between the structure and the reference electrode based on the potential change between the structure and the reference electrode and the applied current change. The optimum potential for protection is determined by electrochemical measurements.
Borna Electronics Co. with over 30 years of experience in the industry, declares its readiness to design, procurement, consultation and construction of anodic protection systems.